
I am an interdisciplinary research fellow, funded by CRUK, working in the groups of Jon Essex, Mark Cragg and Ivo Tews. I am interested in integrating structural biology and computational modelling methods to understand biological activity.
Antibody activity has long been thought to be largely dictated by epitope and affinity. However, antibody dynamics can also play a crucial role. It had been identified that when targetting the tumour necrosis factor receptor (TNFR) CD40, the human IgG2 isotype was more active than the human IgG1 isotype. The key difference between these two isotypes is the antibody hinge region.During my PhD, I investigated the role of the antibody hinge region in controlling antibody dynamics. I used a combination of x-ray crystallography with anomalous scattering, small-angle x-ray scattering (SAXS) and molecular dynamics (MD) simulations to identify that antibodies with increased rigidity gave higher levels of agonism when targetting CD40. I also employed enhanced sampling methods to gain a deeper understanding of the key structural drivers of conformational restriction.1I am now developing this knowledge to further tune antibody agonism and apply the findings to a broader range of key immune receptors.
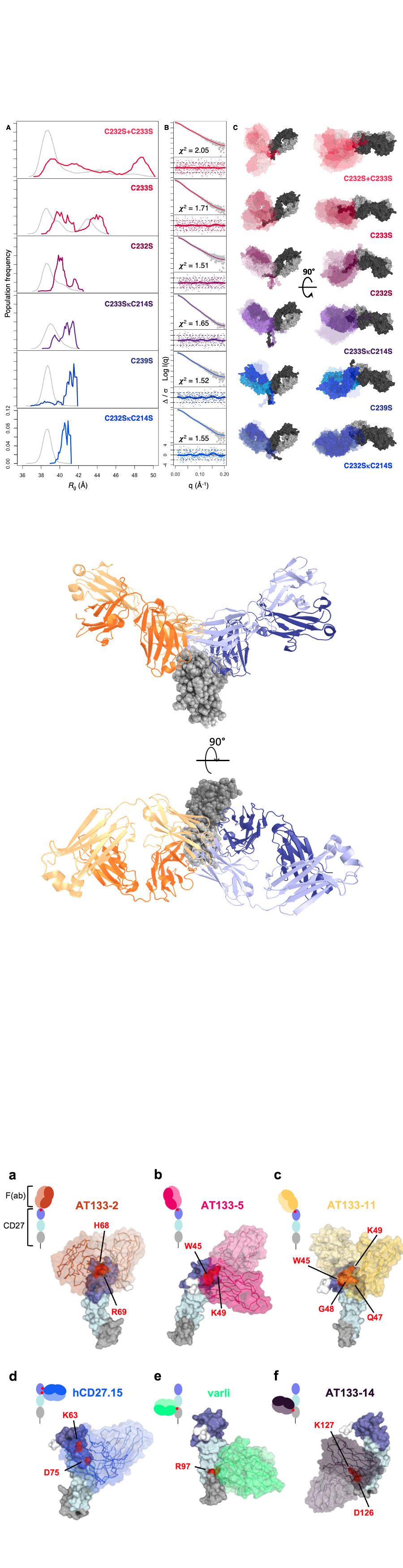
Investigating the interaction of antibodies with their antigens at the atomic level is key to understanding and modifying their activities. Using an integrative structural biology and modelling approach, I have worked on understanding the modes of action of several antibodies targetting a range of immune receptors.CD32bCD32b is a critical immunoregulatory receptor, targeted in the treatment of both cancer and autoimmunity.2 As part of my PhD, I worked on understanding the structural basis for agonism/antagonism for antibodies targetting this key receptor, identifying that a combination of both epitope and affinity were key defining levels of agonism.CD40To understand the role of the epitope in antibodies targetting CD40, I used a combination of data-driven antibody-antigen docking and SAXS to determine models of CD40-Fab complexes.3 Developing on these findings, I am now looking at computational methods to understand and predict how differences in affinity can modulate activity.LILRB3LILRB3 is an inhibitory member of the leukocyte immunoglobulin-like receptor (LILR) family and represents a potential target for cancer immunotherapy.4 In collaboration with the group of Ali Roghanian, I am seeking to elucidate how antibody epitope links to biological activity for this system using a combination of x-ray crystallography, SAXS and cryo-EM.Nectin-4Nectin-4 is a cell surface receptor over-expressed in multiple human malignancies. In collaboration with the group of Sally Ward, I am working to understand the structural modes by which antibodies target nectin 4 in order to develop pH-dependent antibodies.CD27CD27 is a member of the TNFR superfamily acting as a co-stimulatory immune checkpoint molecule. In collaboration with the Lim group, I worked to define the binding epitope of a panel of six antibodies targetting CD27, using a data-driven docking approach.5
It is known that for agonism to occur for anti-CD40 antibodies, clustering of the receptor is key.6 I am now looking at methods to model these cell surface interactions to attempt to understand the key drivers of agonism, whether that be antibody binding epitope, affinity, dynamics or combinations of the three.
I am currently collaborating with the groups of Salah Mansour and Salim Khakoo. With the Mansour group, we are working to engineer 𝛾δ TCR receptors to ensure heterodimer formation during protein refolding. With the Khakoo group, I have worked on understanding the structural basis for differences in affinities in KIR2DS2-peptide-HLA-C complexes.
I am involved in the teaching of molecular visualisation programmes including PyMOL and Chimera to MSci and MRes students.